




|
Key to families and subfamilies of katydids. |
Clypeus: The area of the head between the frons and the labrum.
Epistomal suture: The suture between the frons and the clypeus.
Frons: The area of the head below the arms of the epicranial suture and above the epistomal suture. It bears the median ocellus.
Labrum: The upper lip, which is attached to the clypeus.
Mandibles: The jaws.
Maxillary palp: One of a pair of feeler-like appendages that attach to the mouth parts that are immediately behind the mandibles.
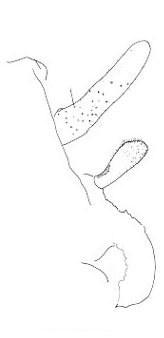
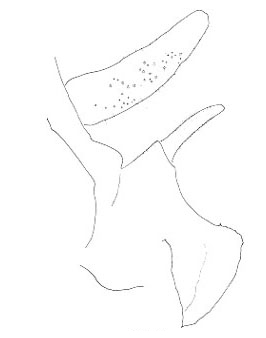
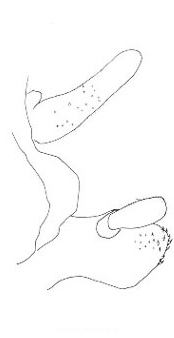
The three North American species are northwestern in distribution and belong to the genus Cyphoderris. The male subgenital plates of C. monstrosa and strepitans (great and sagebrush grigs) have a prominent, ventrally directed process lacking in C. buckelli (Buckell's grig). In C. monstrosa the process is shaped like the nail-pulling claw of a hammer; in C. strepitans it is simple and never terminally cleft.
|
|
|
| sagebrush strepitans |
great monstrosa |
Buckell's buckelli |
Lateral view of male subgenital plates of Cyphoderris.
Fig. 4 from Morris & Gwynne 1978.
C. buckelli and C. monstrosa, the two species that overlap in their geographical distributions, can be distinguished by the pulse rates in their calling songs. At every temperature, C. monstrosa has the higher pulse rate.
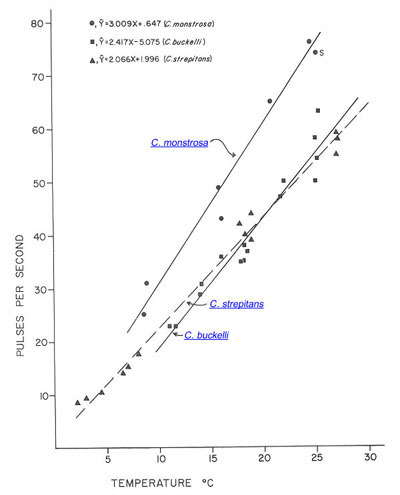
|
Relation of pulse rate and temperature in the calling songs of hump-winged grigs. (S is from Spooner 1973.)
Fig. 8 from Morris & Gwynne 1978 (slightly modified).
Humped-winged grigs are found in coniferous forests; C. strepitans also occurs in high altitude sagebrush. Daylight hours are spent in burrows, and individuals are sometimes collected by turning stones. At night, males produce a succession of short musical trills at wing-stroke rates of 50-75 (at 25°C) and a carrier frequency of about 13 kHz. The songs of C. strepitans and C. buckelli are indistinguishable but the geographical ranges of the two species do not overlap. The right and left forewings of males have equally developed files and scrapers. Either the left or right wing may be uppermost at rest and the two positions occur at approximately equal frequencies (Spooner 1973). The extent to which individual males switch between using the left and right files has not been established. The firmest published evidence of "switch-wing stridulation" is the song of a male C. monstrosa while courting a female (Morris & Gwynne 1978). In this song two pulse types were alternated without break during (in figure below, see bottom oscillogram).
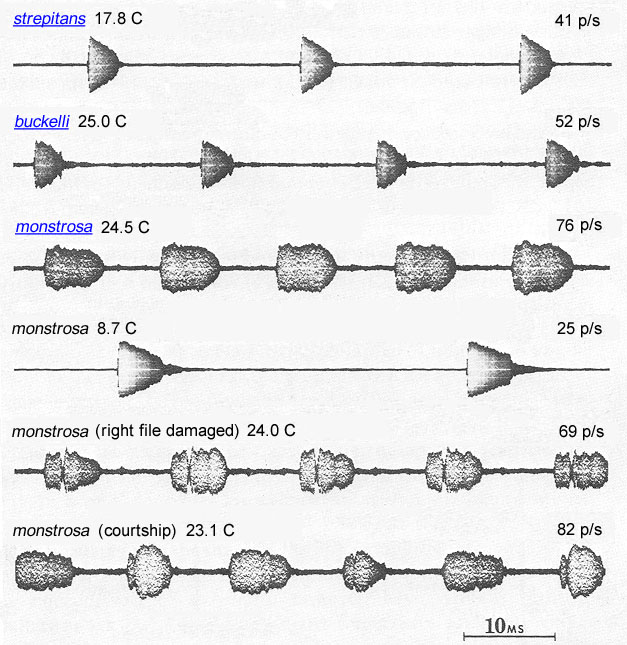
|
Oscillograms of Cyphoderris songs.)
Fig. 6 from Morris & Gwynne 1978 (text added).
During mating, the female feeds on the fleshy hind wings of the male (Morris et al. 1989).
The Prophalangopsidae include only three extant genera: the North American genus Cyphoderris and two Asian genera that are known from fewer than ten specimens from northern India, Tibet, Afghanistan, and extreme eastern USSR. On the other hand, there are many fossil genera of Prophalangopsidae and the related family Haglidae. F.E. Zeuner (1939) recognized 12 fossil genera older than 135 million years and occurring in England, Germany, Turkestan, and South America.
The term grig is a little-used English word for all jumping orthopterans. It is used here (instead of katydid) to emphasize that the split between the Prophalangopsidae and their closest living relatives, the Tettigoniidae, occurred more than 230 million years ago in Permian times (Sharov 1971).
Buckell ER. 1923. Cyphoderris monstrosa Uhler in British Columbia (Orthoptera). Can. Entomol. 55: 225-230.
Dodson GN, Morris GK, Gwynne DT. 1983. Mating behavior of the primitive orthopteran genus Cyphoderris (Haglidae). In: Gwynne DT, Morris GK, editors. Orthopteran mating systems: sexual competition in a diverse group of insects. Boulder CO: Westview. p 305-318.
Eggert AK, Sakaluk SK. 1994. Sexual cannibalism and its relation to male mating success in sagebrush crickets, Cyphoderris strepitans (Haglidae: Orthoptera). Anim. Behav. 47: 1171-1177.
Hebard M. 1934. Studies in Orthoptera which occur in North America north of the Mexican Boundary. II. Cyphoderris, a genus of katydid of southwestern Canada and the northwestern United States. Trans. Am. Entomol. Soc. 59: 371-375. 1 pl.
Johnson JC, Ivy TM, Sakaluk SK. 1999. Female remating propensity contingent on sexual cannibalism in sagebrush crickets, Cyphoderris strepitans: a mechanism of cryptic female choice. Behav. Ecol. 10: 227-233.
Mason AC. 1991. Hearing in a primitive ensiferan: The auditory system of Cyphoderris monstrosa (Orthoptera: Haglidae). J. Comp. Physiol. A. Sens. Neural. Behav. Physiol. 168: 351-364.
Mason AC. 1996. Territoriality and the function of song in the primitive acoustic insect Cyphoderris monstrosa (Orthoptera: Haglidae). Anim. Behav. 51: 211-224.
Mason AC, Morris GK, Hoy RR. 1999. Peripheral frequency mis-match in the primitive ensiferan Cyphoderris monstrosa (Orthoptera: Haglidae). J. Comp. Physiol. A. Sens. Neural. Behav. Physiol. 184: 543-551.
Mesa A, Ferreira A. 1984. A cytogenetic look at the Haglidae through study of the chromosomes of 2 of its 4 relict species Cyphoderris monstrosa and Cyphoderris strepitans (Orthoptera: Ensifera). Occas. Pap. Mus. Zool. Univ. Mich. : 1-11.
Morris GK, DeLuca PA, Norton M, Mason AC. 2002. Calling-song function in male haglids (Orthoptera: Haglidae, Cyphoderris). Can. J. Zool. 80: 271-285. [Cyphoderris buckelli and C. monstrosa]
Morris GK, Gwynne DT. 1978. Geographical distribution and biological observations of Cyphoderris (Orthoptera: Haglidae) with a description of a new species. Psyche 85: 147-166.
Morris GK, Gwynne DT, Klimas DE, Sakaluk SK. 1989. Virgin male mating advantage in a primitive acoustic insect (Orthoptera: Haglidae). J. Insect Behav. 2: 173-185. [Cyphoderris strepitans]
Sakaluk SK, Bangert PJ, Eggert AK, Gack C, Swanson LV. 1995. The gin trap as a device facilitating coercive mating in sagebrush crickets. Proc. R. Soc. Lond. Ser. B. Biol. Sci. 261: 65-71. [Cyphoderris strepitans]
Sakaluk SK, Ivy TM. 1999. Virgin-male mating advantage in sagebrush crickets: differential male competitiveness or non-independent female mate choice? Behaviour 136: 1335-1346.
Sakaluk SK, Morris GK, Snedden WA. 1987. Mating and its effect on acoustic signalling behavior in a primitive orthopteran, Cyphoderris strepitans (Haglidae): The cost of feeding females. Behav. Ecol. Sociobiol. 21: 173-178.
Sakaluk SK, Snedden WA. 1990. Nightly calling durations of male sagebrush crickets, Cyphoderris strepitans: size, mating and seasonal effects. Oikos 57: 153-160.
Sakaluk SK, Snedden WA, Jacobson KA, Eggert AK. 1995. Sexual competition in sagebrush crickets: must males hear calling rivals? Behav. Ecol. 6: 250-257. [Cyphoderris strepitans]
Sharov AG. [1968] 1971. Phylogeny of the Orthopteroidea [translation]. Jerusalem: Israel Program for Scientific Translations. 251 p.
Snedden WA, Sakaluk SK. 1992. Acoustic signalling and its relation to male mating success in sagebrush crickets. Anim. Behav. 44: 633-639. [Cyphoderris strepitans]
Snedden WA, Irazuzta S. 1994. Attraction of female sagebrush crickets to male song: the importance of field bioassays. J. Insect Behav. 7: 233-236. [Cyphoderris strepitans]
Snedden WA. 1996. Lifetime mating success in male sagebrush crickets: sexual selection constrained by a virgin male mating advantage. Anim. Behav. 51: 1119-1125. [Cyphoderris strepitans]
Spooner JD. 1973. Sound production in Cyphoderris monstrosa (Orthoptera: Prophalangopsidae). Ann Entomol Soc Am 66: 4-5.
Willey RB, Willey RL. 1963. Range extension of Colorado Cyphoderris (Orthoptera: Prophalangopsidae). Entomol. News 75: 200-201.
Zeuner FE. 1939. Fossil Orthoptera Ensifera. London: British Museum Natural History. 321 p.